Discover
Quickly identify potential participants anonymously and efficiently produce a list for IRB approval.
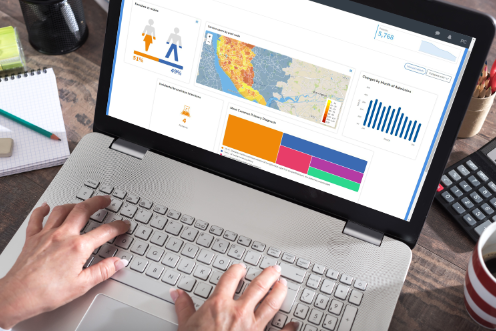
Atmolytics accelerates the identification of qualified candidates, increasing the number of clinical trials and research studies.





Quickly identify potential participants anonymously and efficiently produce a list for IRB approval.

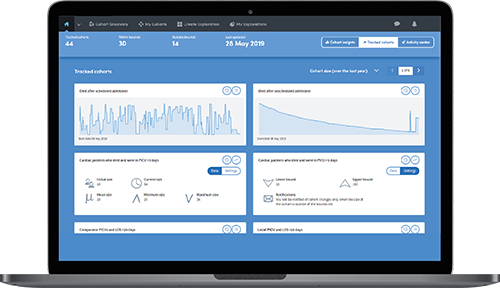
Track eligible candidates over time and receive daily emails about changes in size, joiners and leavers.
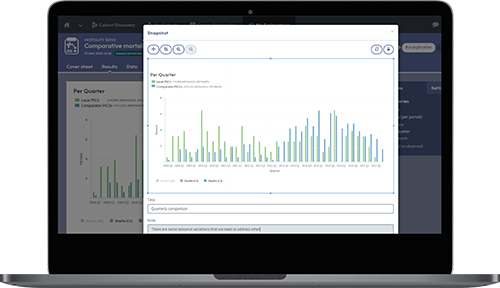
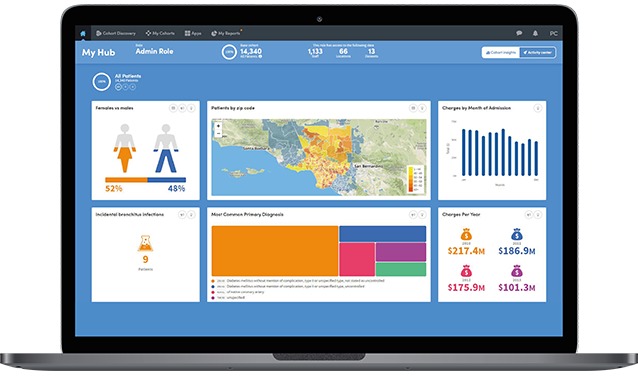
Snapshots can be taken of key results which can then be shared and discussed across teams.

For greater transparency you can see detailed views of the steps applied against the data that meets the criteria for candidate identification.
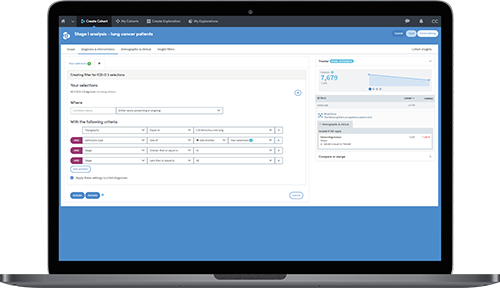
A cancer research center has implemented Atmolytics to support an 'Honest Broker' system which is speeding up the ability of researchers and principal investigators to identify patients who meet the criteria for a clinical study or trial.